COVID-19-VAED: Spike Protein Disease
The vaccines alone cause what the CDC describe as vaccine-enhanced disease
Summary
The CDC list a number of disease processes that "could indicate vaccine-enhanced [COVID-19] disease", all of which can manifest both as side effects of the COVID-19 vaccines and as complications of COVID-19 itself.
Their reported proportions among VAERS reports are highest in reports about double-dosed patients who suffer a SARS-COV-2 infection and lowest in reports about single-dosed patients who did not report being exposed to SARS-COV-2.
Since the only common denominator of SARS-COV-2 and the COVID-19 nucleotide-based vaccines is their ability to stimulate translation of spike protein in the cells they enter, I determined the reported proportions of nine medical concepts that I consider features of spike protein associated disease in report subsamples defined by dose series and infection status.
Spike protein associated disease features were reported at a significantly higher proportion (p<0.0001) in double-dosed individuals compared to single-dosed individuals (PRR 2.02, 99.99% CI: 1.87-2.17) and at a significantly higher proportion (p<0.0001) in SARS-COV-2-exposed individuals compared to individuals not reportedly exposed to the SARS-COV-2 (PRR 2.68, 99.99% CI: 2.43-2.96).
Introduction
Vaccine-enhanced disease (VAED) has been demonstrated in various scenarios in the past. Its features can not easily be generalized and have so far eluded a clear definition. There seem to be several distinct mechanisms which lead to disease modification that are understood as aspects of VAED.
In an earlier article we looked at the occurrance of myocarditis as a feature of vaccine-enhanced disease after the CDC had suggested on January 29th 2021 that the occurrance of myocarditis could indicate VAED.
In the same document, the CDC suggest additional types of adverse events that deserve attention because they could indicate VAED:
Coagulopathy (Thrombocytopenia, Veinous thromboembolisms, Disseminated intravascular coagulation)
Multisystem Inflammatory Syndrome in Children (MIS-C) and in Adults (MIS-A)
The CDC further list types of adverse events that they do not consider indicators of VAED:
I looked at the report proportions of various MedDRA terms that describe these adverse events of special interests in four subsamples of reports:
Reports about single-dosed individuals
Reports about double-dosed individuals
Reports about individuals reporting virus exposure
Reports about individuals not reporting virus exposure
This allows me to identify processes,
…that occur as a result of vaccination in patients without reported SARS-COV-2 exposure.
…that are more commonly reported by double-dosed than by single-dosed individuals.
…that are more commonly reported by individuals who report being exposed to SARS-COV-2 than by those who do not report virus-exposure.
I understand processes fulfilling these criteria as the result of spike protein expression by cells that were either transfected through vaccination against COVID-19 or infected with SARS-COV-2.
Methods
I supply a brief update to my earlier article about the occurrance of myocarditis in children aged 12 or older, that aims to satisfy the CDC definitions of suspected and probable myocarditis as well as include those cases that were reported as diagnosed myocarditis.
Each type of myocarditis is defined by several requirements relating to the inclusion of certain MedDRA terms in the reports:
Suspected myocarditis
ANY: Dyspnoea / Palpitations / Chest pain
NOT: Myocarditis / Troponin increased
Probable myocarditis
ANY: Dyspnoea / Palpitations / Chest pain
HAS: Troponin increased
NOT:Myocarditis
Diagnosed myocarditis
HAS: Myocarditis
Any myocarditis
ANY: Suspected / probable / diagnosed myocarditis
The following medical concepts serve to define the other types of adverse events of special interest:
Indicators of VAED in individuals not reportedly virus-exposed
Acute myocardial infarction
Coagulopathy
Myopericarditis
Stroke
MIS
MIS (Multisystem inflammatory syndrome in adults / Multisystem inflammatory syndrome in children / Multisystem inflammatory syndrome)
Any VAED indicator (any occurrance of 1. - 9.)
Indicators of VAED only reported by virus-exposed individuals
Severe course of COVID-19 disease
Hospitalisation (or HOSPITAL=1)
Severe COVID-19 (any occurrance of 11. - 13.)
Events not associated with VAED according to the CDC
Anaphylaxis
Appendicitis
Bell’s Palsy
GBS (Guillain-Barré Syndrome)
Seizure
Report cohorts for all other terms are defined as follows:
Single-dosed,
Double-dosed,
Virus-exposed,
Not reportedly virus-exposed
Reports about single-dosed individuals have “1” specified as maximum value in the DOSE_SERIES field for COVID-19 vaccines
Reports about double-dosed individuals have “2” specified as maximum value in the DOSE_SERIES field for COVID-19 vaccines
Reports about virus-exposed individuals list at least one of the MedDRA terms Vaccine breakthrough infection, COVID-19, SARS-CoV-2 test positive, COVID-19 pneumonia, Asymptomatic COVID-19, Post-acute COVID-19 syndrome
All proportions referring to the various types of myocarditis refer only to reports about events occuring in boys aged 12-17 within 30 days of vaccination (Figure 1)
All other proportions of all other terms do not consider patient age, gender or temporal distance to vaccination (Figure 2ff.)
Reports that do not specify patient age or gender have been classified as unreliable and have thus not been included in any of the report cohorts.
All proportions for all relevant MedDRA terms and all report cohorts are displayed on a few simple bar charts.
To gain an impression of the impact of repeated exposure to spike protein, the ratios of reported proportions and the respective 99.99% confidence intervals are calculated and plotted for:
Second transfection: Proportion ratio between
events occurring after 2 doses
events occurring after 1 dose
Infection: Proportion ratio between
reports about virus-exposed individuals
reports about individuals not reportedly virus-exposed
Results
Myocarditis in males aged 12-17
Almost 1 out of 4 boys who reported being exposed to SARS-COV-2 within 30 days of receiving their second dose of a COVID-19 vaccine were at least suspected to have suffered myocarditis.
These proportions are very close to the incidence proportions determined by a cohort study conducted in Thailand.
Other adverse events (proportions)
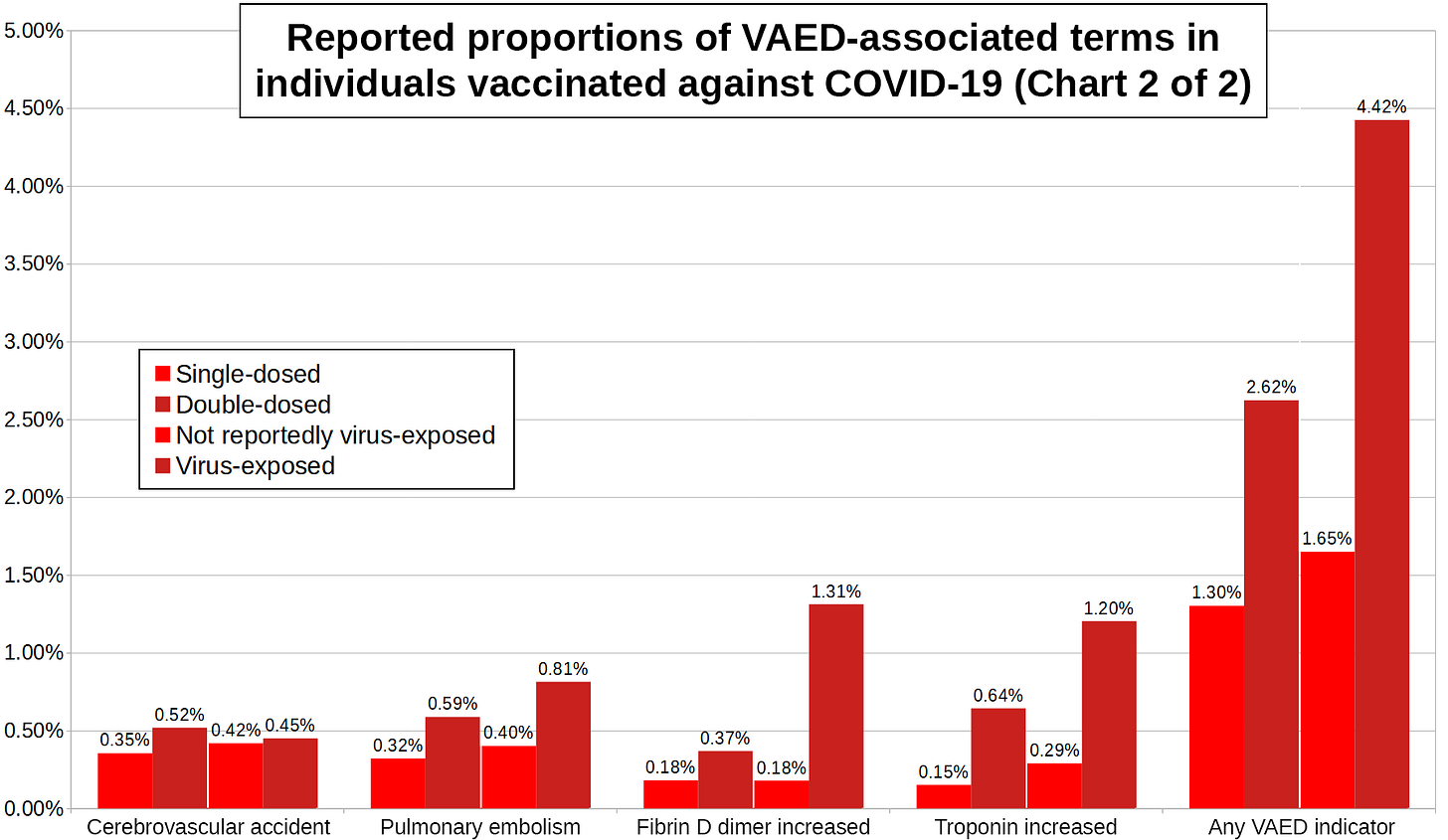
All VAED-associated concepts’ report proportions are higher…
…among reports about double-dosed individuals than among reports about single-dosed individuals,
…among reports about virus-exposed individuals than among reports about individuals not reportedly virus-exposed.
All report proportions of severe COVID-19 disease features are higher among reports about double-dosed individuals who were exposed to SARS-COV-2 than among reports about single-dosed individuals who were exposed to SARS-COV-2.
These patterns can not be observed in those types of adverse events of special interest that the CDC don’t consider indicators of VAED.
Neither are these events reliably reported at higher proportions after dose 2 compared to dose 1, nor are any of them reported at the same or at a higher proportion in SARS-COV-2-exposed individuals compared to individuals not reportedly exposed to SARS-COV-2.
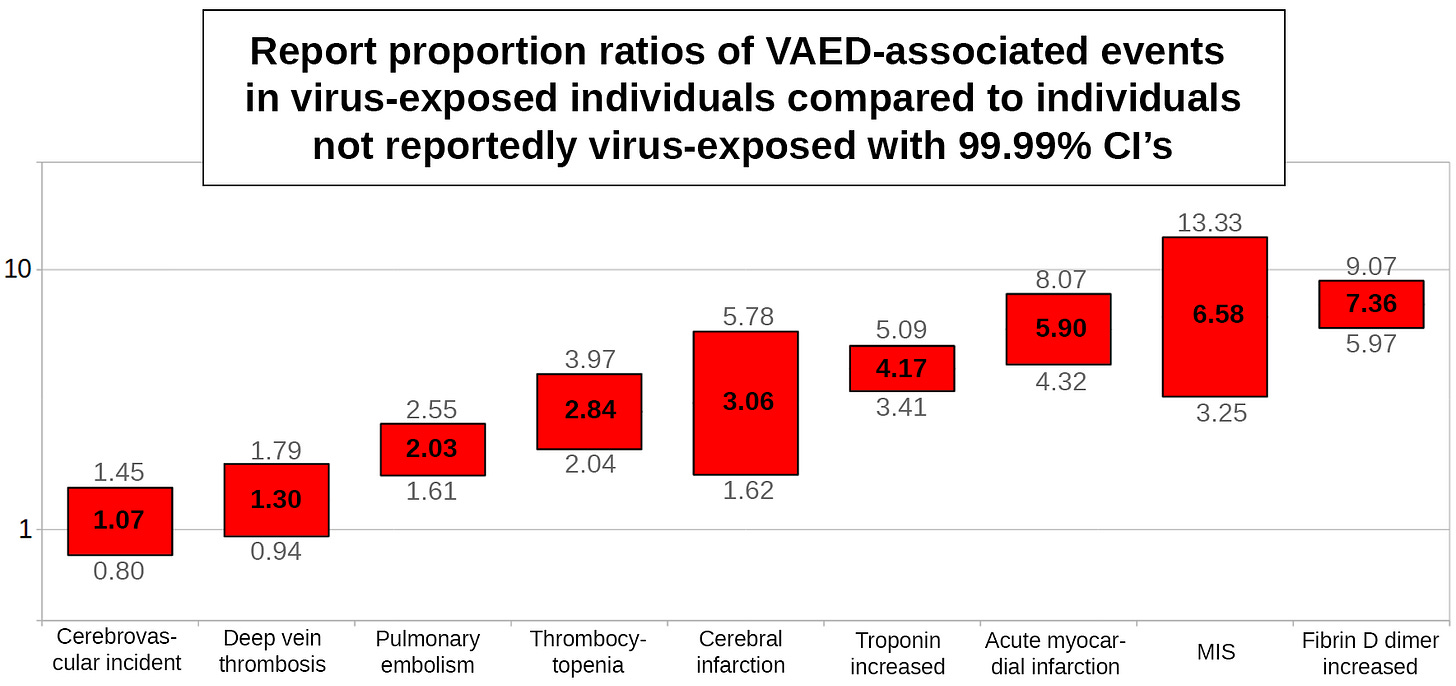
VAED-associated events (proportion ratios)
All 9 VAED-associated events are reported at higher proportions among individuals who reported SARS-COV-2 exposure compared to individuals not reportedly exposed to SARS-COV-2. They can all apparently occur as complications of SARS-COV-2 infection.
Out of 549,411 reports that do not mention SARS-COV-2 exposure, 9,060 (1.65%) contain at least one of the nine terms, while this is the case for 1,788 out of a 40,422 (4.42%) reports about individuals who were exposed to SARS-COV-2.
The proportional ratio of VAED-associated events between virus-exposed individuals and individuals not reportedly virus-exposed is 2.68 (99.99% CI: 2.43-2.96).
All 9 VAED-associated events are reported at higher proportions among individuals who received 2 doses compared to individuals who only received 1 dose.
Out of 349,760 reports about single-dosed individuals, 4,552 (1.30%) contain at least one of the nine terms, while this is the case for 6,296 out of a 240,073 (2.62%) reports about double-dosed individuals.
The proportional ratio of VAED-associated events between double-dosed and single-dosed individuals is 2.02 (99.99% CI: 1.87-2.17).
COVID-19 pneumonia, death and hospitalisation are all reported at higher proportions among double-dosed infected compared to single-dosed infected individuals.
Out of 12,976 reports about infections in single-dosed individuals, 4,616 (35.57%) describe a severe course of disease, while this is the case for 15,886 out of a 27,446 (57.88%) reports about double-dosed individuals.
The proportional ratio of severe COVID-19 between double-dosed and single-dosed individuals who reported infections is 1.63 (99.99% CI: 1.59-1.67).
Conclusion
There are inflammatory (MIS, Myocarditis) and coagulative (7 remaining terms) disease processes associated with the expression of spike protein. These processes occur both as complications of COVID-19 itself (p<0.0001) and as side effects of nucleotide-based vaccination against the disease (p<0.0001):
The proportional ratio of VAED-associated events between virus-exposed individuals and individuals not reportedly virus-exposed is 2.68 (99.99% CI: 2.43-2.96).
The proportional ratio of VAED-associated events between double-dosed and single-dosed individuals is 2.02 (99.99% CI: 1.87-2.17).
Among reports about infections, a much higher proportion of double-dosed individuals report severe disease course (57.88%) compared to single-dosed individuals (35.57%):
The proportional ratio of severe COVID-19 between double-dosed and single-dosed individuals who reported infections is 1.63 (99.99% CI: 1.59-1.67).
These report proportions supply empirical evidence that COVID-19 vaccines based on spike protein encoding nucleotide sequences enhance COVID-19 disease.
Discussion
To understand how VAERS data can suggest that the vaccines enhance disease, while other data suggest a reduction in risk for severe disease course, one has to look at how the number of reported infections change with increasing temporal distance to the time of vaccination, which I am planning to do in my next article.